Everyone knows that water is vital
for life. Space probes look for evidence of water on other planets and moons as
a sign that these may support life. What are the physical properties of water
that make it so amazing?
Liquid water
Water has a large temperature range (100
degrees) for its liquid state. Other liquids that may be in the solar system do
not have such a range. Ammonia is common, but its liquid range is 44.4 degrees
(-77.7 to -33.3 °C). Methane is liquid for only 20
degrees (-182.5 to -161.5 °C).
Liquids are important for life
because chemicals dissolved in a liquid can move freely while relatively close
to each other. This allows the chemical reactions needed for life to occur
inside our cells.
A powerful solvent
Water is the main ingredient in many
cleaning products because it is a powerful solvent. The uneven sharing of
electrons between the oxygen and hydrogen make water a polar molecule. The
slight charges on different sides of the water molecule allow it to break apart
solids and surround each constituent molecule. This is how a solid dissolves.
The molecules of life are dissolved
in cells. DNA, RNA, proteins and carbohydrates react with each other in an
aqueous solution. Gases and nutrients dissolved in our blood are transported
through our body. Water carries micronutrients and sugars through the transport
systems in plants.
The
chemicals of life are dissolved and react with each other inside our cells.
Cold water sinks, but ice floats
The solid form of a substance
generally has a higher density than the liquid. This means that solids sink.
You can see this when you melt butter – the solid butter remains at the bottom
of the pan. Water is different because the solid form (ice) is less dense than
liquid. Lower density is why ice cubes float in a glass of water.
Floating ice is very important for
life on Earth. When bodies of water freeze on top, the liquid below can still
support life and is insulated from temperature changes in the air. Water is
most dense at 4°C. Cold water sinks due to gravity,
causing ocean currents which move water around the planet and bring nutrients
up from the depths of the oceans. This effect is enhanced by dissolved salts.
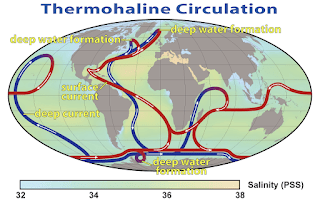
The global movement of water is called thermohaline circulation.
Cold,
salty water is more dense (blue) and flows in deep currents that rise to the
surface in areas of upwelling. The thermohaline circulation distributes heat around
the planet. (R Simmon, NASA 2008, public domain)
Specific heat capacity
Specific heat capacity is a
measurement of the amount of energy needed to heat up a substance. Water has a
high specific heat. It takes much more energy to raise the temperature of a
gram of water than it does to raise the temperature of a gram of metal or sand.
This means that the Sun’s energy changes the temperature of the oceans very
gradually. Thus, water provides a stable environment for life.
Because of its high specific heat, water
affects climate. The stability of ocean temperatures results in much lower
temperature variation in coastal areas, compared with inland areas. When the
trade winds change, the El Niño Southern Oscillation changes. This
greatly affects the climate in Australia and South America during its extreme
forms.
The oceans offer a stable
environment for life.
Explore wonderful water
- Learn more about the properties of water in the AusEarthEd videos Wonderful Water 1: Basic Properties and Wonderful Water 2: Specific Heat.
- Explore ocean currents with WASP activities and information.
- Experiment with thermohaline currents as shown in the video and learn more about the Global Conveyor Belt in the blog post.