You might be
surprised to learn that the concept of using hydrogen for transportation is not
new. As far back as 2004, Perth conducted a three-year trial using three Mercedes-Benz
hydrogen fuel-cell "Ecobuses". This trial was part of an
international project called "CUTE"
(Clean Urban Transport for Europe) and "ECTOS"
(Ecological City Transport System), which also included other cities such as Amsterdam,
Barcelona, Beijing, Hamburg, London, Luxemburg, Madrid, Oporto, Reykjavik,
Stockholm, and Stuttgart.
So how do these
hydrogen-powered vehicles work? Each Ecobus used two Proton Exchange Membrane
(PEM) fuel cells developed by a Canadian company called Ballard. A fuel cell
is a device that uses hydrogen (or hydrogen-rich fuel) and oxygen to generate
electricity. If pure hydrogen is used as the fuel, these fuel cells only
produce heat and water as byproducts, which means they don't
contribute to air pollution or greenhouse gas emissions. This type of fuel
cell, known as a PEM fuel cell, is still the most common one used today.
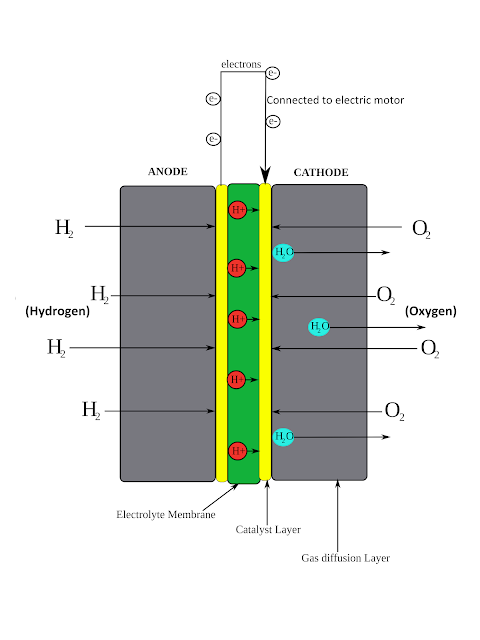
Let’s break down the components of a PEM fuel cell:
- PEM (Proton Exchange Membrane) - This is a thin, solid, organic compound that allows protons to pass through but not electrons. It needs to stay moist to function properly.
- Anode: The anode is negatively charged and made up of platinum particles supported on carbon particles. It acts as a catalyst to speed up the chemical reaction (oxidation) and allows hydrogen to pass through it.
- Cathode: The cathode is positively charged and composed of platinum particles supported on carbon particles. It also acts as a catalyst, but for a different chemical reaction (reduction). It allows oxygen to pass through it.
- Flow Plates: These plates have several essential functions. They direct hydrogen and oxygen to the electrodes, remove water and excess heat from the fuel cell, and conduct electrons from the anode to the electrical circuit and back to the cathode.
Here's how the chemical process works in a PEM fuel cell:
- H2 and Anode - Hydrogen fuel (H2) is supplied to the anode, where a catalyst separates the hydrogen's negatively charged electrons from the positively charged protons.
- PEM - The membrane allows the positively charged protons to move through to the cathode while blocking the negatively charged electrons.
- Cathode - On the cathode side, oxygen gas (O2) is forced through the catalyst, splitting it into two oxygen atoms with a strong negative charge. This charge attracts the protons through the membrane, where they combine with an oxygen atom and two electrons from the external circuit to form water (H2O).
Let's look at the positives and challenges of hydrogen fuel cells.
Positives:
- Zero Emissions - Hydrogen-powered fuel cell electric vehicles only emit water (H2O) and warm air. into the atmosphere.
- Energy security - Hydrogen can be produced domestically using resources like natural gas, biomass, solar, and wind energy. When used in highly efficient fuel cell electric vehicles, hydrogen can help enhance national energy security, reduce reliance on petroleum, and offer additional transportation energy options for a more resilient system.
- Greenhouse Gas Reduction - By using "green hydrogen," which is produced from renewable sources, the entire process of hydrogen production and use can have minimal greenhouse gas emissions.
- Helps Australia meet emission targets - By using a low greenhouse gas emitting fuel Australia can more easily meet its greenhouse gas reduction target of Net Zero by 2050.
Challenges:
- Greenhouse Gas Production - Hydrogen produced from crude oil or natural gas still contributes to greenhouse gas emissions.
- Energy Density and Storage - Hydrogen has a low energy content by volume, making storage a challenge. It requires high pressures, low temperatures, or chemical processes to store it compactly. This is especially important for light-duty vehicles with limited space and weight capacity for fuel storage.
- Cost and Infrastructure - To be competitive in the market, the cost of fuel cells needs to decrease significantly without compromising performance. However, experts predict that mass-produced fuel cell electric vehicles could have similar costs to hybrid vehicles by 2025. Additionally, the cost of building and maintaining hydrogen stations needs to decrease for a viable hydrogen economy. This is like the current challenge of installing enough electric vehicle charging stations, especially in areas with a low and widely dispersed population like Western Australia.
Exploring alternatives to petrol and diesel vehicles is crucial for reducing greenhouse gas emissions and mitigating air pollution. Battery-powered electric vehicles have gained popularity, but hydrogen fuel cells also offer a promising solution. By understanding how hydrogen fuel cells work and considering their pros and cons, we can make informed decisions about the future of transportation and sustainable energy.
References
Office of Energy Efficiency and
Renewable Energy
Bus Preservation
Society of WA
Hydrogen Fuel Cell Buses